The periodic table is a cornerstone of chemistry, arranging elements based on their properties and atomic structure. In this comprehensive guide, we’ll delve deeper into the intricacies of the periodic table, exploring its definition, historical development, and importance in the world of science.
Understanding the Periodic Table
The periodic table is a tabular arrangement of chemical elements based on their atomic numbers, electron configurations, and periodic chemical properties. It provides a systematic framework for understanding the relationships between different elements and predicting their chemical behaviour.
Historical Development
Early Attempt:
The concept of organizing the elements dates back to ancient times when scholars speculated about the fundamental building blocks of matter. However, significant progress was not made in this field until the 19th century.
Mendeleev’s Contribution:
In 1869, Russian chemist Dmitri Mendeleev proposed the first version of the periodic table. He arranged the elements in order of increasing atomic weight and grouped them based on similar properties. Mendeleev’s table also left gaps for undiscovered elements, predicting their properties with remarkable accuracy.
Modern Periodic Table:
The modern periodic table, as we know it today, has undergone many revisions and refinements since Mendeleev’s time. With advances in atomic theory and experimental techniques, scientists have gained a deeper understanding of atomic structure and fundamental properties, leading to the development of the current periodic table.
About the Old Periodic Table
The old periodic table stands as a fundamental tool in the field of chemistry, serving as a roadmap to the elements that make up matter. In this article, we will delve deeper into the definition of the old periodic table, trace its origins, clarify its structure, and discover its importance in the scientific community.
The old periodic table refers to earlier iterations of the periodic table, which predate the modern version we are familiar with today. While the modern periodic table boasts a sophisticated structure and organization, the older periodic tables laid the foundation for our understanding of the chemical elements and their properties.
Origin and Development
Mendeleev’s Contribution:
One of the most notable figures in the development of the periodic table is the Russian chemist Dmitri Mendeleev, who proposed the first version of the periodic table in 1869. Mendeleev arranged the elements in order of increasing atomic weight and grouped them based on similarity. His table also left gaps for undiscovered elements, predicting their properties with remarkable accuracy.
Limitations and Amendments:
While Mendeleev’s periodic table was a groundbreaking achievement, it had its limitations. As scientific knowledge advanced and new elements were discovered, revisions to the periodic table became necessary. Over time, scientists refined the structure of the periodic table, incorporating new elements and adjusting the arrangement based on emerging trends and principles of atomic theory.
Structure and Organization
The old periodic table generally arranged the elements in rows and columns, with each element represented by its atomic symbol and atomic weight. Elements were grouped based on similarities in their chemical properties, allowing scientists to identify patterns and trends among different elements.
Significance and Legacy
Despite its ancient nature, the Old Periodic Table holds immense importance in the history of science. It laid the foundation for our modern understanding of chemical elements and their interactions, leading to unprecedented discoveries in chemistry and related fields.
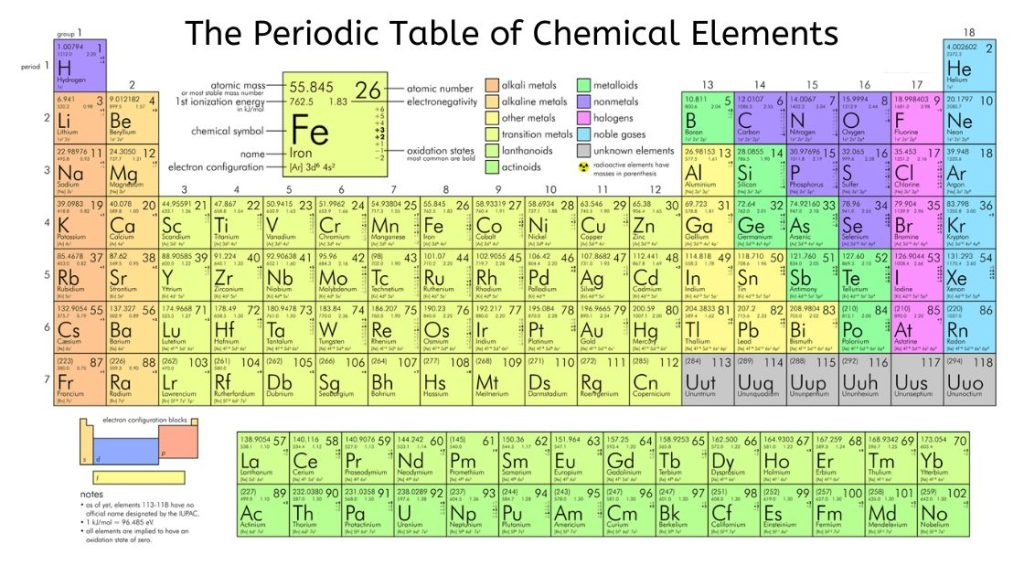
About the Modern Periodic Table
The modern periodic table stands as a cornerstone of chemistry, providing a systematic framework for understanding the properties and behaviour of chemical elements. In this comprehensive guide, we’ll dive deep into the definition of the modern periodic table, explore its structure, and highlight its importance in the world of science.
The modern periodic table is a tabular arrangement of chemical elements based on their atomic numbers, electron configurations, and periodic chemical properties. Unlike its predecessors, the modern periodic table provides a more sophisticated and comprehensive understanding of the relationships between different elements.
Development of the Periodic Table
Mendeleev’s Contribution:
The modern periodic table is built on the foundation laid by the Russian chemist Dmitri Mendeleev, who proposed the first version of the periodic table in 1869. Mendeleev arranged the elements in order of increasing atomic weight and grouped them based on similar properties. His table also left gaps for undiscovered elements, predicting their properties with remarkable accuracy.
Moseley’s Progress:
In 1913, British physicist Henry Moseley introduced the concept of atomic number, which replaced atomic weight as the primary criterion for arranging elements. This success led to the development of the modern periodic table, which arranges the elements in order of increasing atomic number from Hydrogen (atomic number 1) to Oganesson (atomic number 118).
Structure of the Modern Periodic Table
The modern periodic table consists of rows called periods and columns called groups. Each period represents a new energy level or shell of electrons, while each group shares the same electron configuration and chemical behaviour.
Periods:
- There are a total of 7 periods in the modern periodic table, each of which corresponds to a different principal quantum number or energy level.
- As you move from left to right across a period, the number of electrons in the outermost shell increases, resulting in predictable changes in atomic properties.
Group:
- There are 18 groups in the modern periodic table, each represented by a number and often named after the first element in the group.
- Elements in the same group share similar chemical properties due to their similar valence electron configurations.
Importance of Modern Periodic Table
The modern periodic table holds immense importance in the field of chemistry for several reasons:
Organization and Predictability:
- It provides a systematic arrangement of elements, making it easier to study and understand their properties and relationships.
- Scientists can predict the properties of undiscovered elements based on their positions within the table, facilitating research and experimentation.
Classification and Trends:
- Elements are classified and periods based on their atomic structure and properties, which provide valuable insight into their behaviour and reactivity.
- Periodic trends such as atomic radius, ionization energy, and electronegativity can be observed and analyzed within the framework of the periodic table.
Classification of Elements in the Periodic Table
The periodic table is a fundamental tool in chemistry, organizing the vast array of chemical elements into a systematic framework. In this article, we will explore the classification of elements, the principles behind their arrangement, and the importance of different groups within the periodic table.
Basis of Classification
Atomic Number:
The primary criterion for classifying elements in the periodic table is their atomic number, which represents the number of protons in the nucleus of the atom. The elements are arranged in order of increasing atomic number from Hydrogen (atomic number 1) to Oganesson (atomic number 118).
Period and Group:
Elements are arranged into Periods (Horizontal Rows) and Groups (Vertical Columns) based on similar properties. Each period represents a new energy level or shell of electrons, while each group shares the same electron configuration and chemical behaviour.
Classification by Groups
Alkali Metals (Group 1):
Alkali metals, located in Group 1 of the periodic table, include elements such as lithium, sodium and potassium. They are highly reactive metals, often combining with water to form alkaline solutions.
Alkaline Earth Metals (Group 2):
Group 2 elements, including magnesium, calcium, and barium, are known as the alkaline earth metals. They exhibit similar properties to the alkali metals but are slightly less reactive.
Transition Metals (Groups 3-12):
Transition metals occupy the central part of the periodic table, including elements such as iron, copper, and gold. They are characterized by their variable oxidation states and often serve as catalysts in chemical reactions.
Halogens (Group 17):
Group 17 elements, including fluorine, chlorine, and bromine, are known as halogens. They are highly reactive nonmetals, often found in salt-forming compounds.
Noble Gases (Group 18):
Noble gases like helium, neon and argon are in group 18 of the periodic table. They are inert, meaning they have stable electron configurations and exhibit minimal reactivity.
Periodic Trends
Atomic Radius:
Atomic radius generally decreases from left to right across a period due to increasing nuclear charge, while it increases down a group due to additional electron shells.
Ionization Energy:
Ionization energy increases across a period and decreases across a group, reflecting the ease with which an atom can lose or gain electrons.
Electronegativity:
Electronegativity follows the same trend as ionization energy, increasing across a period and decreasing across a group.
Must Watch
Importance of the Periodic Table
The periodic table holds immense importance in the field of chemistry for several reasons:
- Organization of Elements: It provides a systematic arrangement of elements, making it easier to study and understand their properties and relationships.
- Estimated Power: The periodic table allows scientists to predict the properties of undiscovered elements based on their position within the table, facilitating research and experimentation.
- Classification of Elements: Elements are classified and periods based on their atomic structure and properties, which provide valuable insight into their behaviour and reactivity.
- Chemical Research Foundation: The periodic table serves as the foundation of various branches of chemistry, including inorganic, organic, and physical chemistry, guiding research and discovery in these areas.
Conclusion
Ultimately, the periodic table stands as a testament to humanity’s quest for knowledge and understanding of the natural world. From its humble beginnings to its modern iteration, the periodic table has revolutionized the field of chemistry, shaping our understanding of the elements and their interactions. As we continue to unravel the mysteries of the universe, the periodic table remains an indispensable tool, guiding scientists on their journey of discovery and discovery.
FAQs
Question: How many total elements are there in the periodic table?
Answer: As per the periodic law, the properties of Elements are periodic functions of their atomic numbers. The Periodic Table is made up of 118 Elements.
Question: Who invented the periodic table?
Answer: Dmitri Mendeleev.